Imaging-Guided Transcranial Direct Current Stimulation (tDCS) in Major Depression
UCLA Clinical Research Study (IRB #20-001544)
We are recruiting participants for a research study conducted at UCLA. Research studies are voluntary and include only people who choose to take part, and who meet all of the eligibility criteria as determined by the study investigators.
Study Description
Transcranial direct current stimulation (tDCS) is a non-invasive method of stimulation that uses electrodes placed on the scalp to deliver a constant, low current. This study is being done to examine how tDCS affects the brain, and if brain changes are related to changes in mood. To see how tDCS affects brain signals, this study will use MRI scans.
To make sure that changes are because of tDCS and not for other reasons, people will be randomized (assigned by chance) to receive either active or non-active (sham) tDCS using standard or small (high definition, HD) electrodes. This means if you agree to participate, you might not receive active experimental tDCS. You will be told at the end of the study if this is the case. If you did not receive active tDCS during the research, you will be invited to receive the same number of active tDCS sessions after the study. You may decide or not decide to receive these additional active tDCS sessions after your research participation is complete.
We aim to enroll 100 patients who have depression and who have not made any changes to current medication(s) for the past six weeks.
If you are eligible, your participation in the research may last approximately two months. During this time, you will be required to make 13 separate visits to UCLA and complete two brief mood assessments over the phone 2 and 4 weeks after your final in-person visit. Complete research study details can be found here.
This research study (R33MH110526) is being conducted with support from the National Institute of Mental Health (NIMH). We will give access to some of the de-identified data, including MRI images and most behavioral data, to the scientific community through the NIMH Data Archive. We will make all of the data available to other investigators that have been approved by the study researchers. Your data will be coded and will NOT have your name on it or any other personally identifying information. Letting us use and share your data is voluntary. However, you must be willing to share your data in this way in order to participate in this study. If you are not willing, you cannot participate in this research study.
Eligibility Requirements:
- Currently diagnosed with depression.
- Have not made any changes to current medications for the past 6 weeks.
- Are between 18-65 years of age.
- Are willing to receive several MRI scans.
- Are able to comfortably wear an electrode cap.
- Refrain from making any drastic hair changes over the course of the study to ensure that the electrode cap has a consistent fit throughout.
- Live within driving distance to UCLA, and are able to come in daily (excluding weekends) for tDCS.
Process for determining eligibility:
- Initial eligibility will be determined by speaking with a study coordinator by phone, who will ask you questions about your medical history.
- If it appears that you may be eligible for the research study, we can schedule your consult visits to determine eligibility.
- Note – if you are currently taking benzodiazepines or sleeping pills, you must refrain from taking these for the duration of the study.
- If, after the initial Consult visits, you meet all eligibility criteria, a study coordinator will schedule all of your remaining visits as soon as our schedule allows.
The researchers may end your participation in the study for a number of reasons, such as if your safety and welfare are at risk, if you do not follow instructions or if you miss scheduled visits.
If you are interested in speaking with a study coordinator to find out if you are potentially eligible for the study:
Please email DGCNeurostimStudy@mednet.ucla.edu or call 424-402-9051 to speak to the Study Coordinator.
Research Study Details
If you are eligible, your participation in the research will last approximately two months. During this time, you will be required to make 13 separate visits to UCLA and complete two brief mood assessments over the phone 2 and 4 weeks after your final in-person visit.
- To determine if you are potentially eligible to participate in the study, you must first speak with a study coordinator by phone who will ask you questions about your medical history. Time: Approximately 15 minutes.
- If it appears that you may be eligible for the research study, we can schedule your initial appointments (Consult visits) to determine eligibility. If you are currently taking benzodiazepines or sleeping pills, you will need to refrain from taking these for the duration of the study. If your treating physician indicates that it is not safe for you to taper these medications before beginning the trial, you will not be eligible to participate.
- Consult: : the first visit to determine eligibility will be divided into two parts. The first part will be completed remotely and will include a psychiatric evaluation and detailed clinical mood evaluations. At the start of this visit you will be asked to sign the research consent form. During the second part of the Consult, you will be asked to come to UCLA to receive a brain MRI scan and to complete some. cognitive tests of thinking and memory on a computer . The study doctors will need to review the results of these tests before deciding if you qualify for receiving tDCS. Time: Approximately 5 hours.
- Note that the evaluation visit will last approximately 3 hours and the MRI appointment will last approximately 2 hours.
- If you qualify, a study coordinator will schedule all of your remaining visits as soon as our schedule allows.
- The remaining visits will occur approximately one week after your Consult visit and will be scheduled to occur on 12 consecutive days (excluding weekends).
- These visits are based on the study physician, MRI scanner, and clinic availability, which are limited. Therefore, we do not have a lot of flexibility.
- If you do not qualify, you will be notified by a study coordinator. As this is a research study, we have strict eligibility requirements, and exceptions cannot be made.
- Baseline Assessment: : in your second visit you will complete some cognitive testing (if not already completed as part of the consult visit) and mood evaluations, be prepared for tDCS administration, and receive a functional MRI scan lasting approximately 1 hour. During part of this scan, tDCS will be delivered.Time: Approximately 4 hours.
- Visits 3-6: : in the next four consecutive visits, you will be prepared for tDCS administration and receive 20 minutes of tDCS in a private room. Time: Approximately 1 hour.
- Visit 7: you will return for visit 7, during which you will be prepared for tDCS administration, receive 20 minutes of tDCS in a private room, and complete mood assessments with study staff. Time: Approximately 3.5 hours.
- Visits 8-12: in the next five consecutive visits, you will be prepared for tDCS administration and receive 20 minutes of tDCS in a private room. Time: Approximately 1 hour.
- Post-Trial Assessment: you will return for a post-trial assessment, during which you will be prepared for tDCS administration and receive a functional MRI scan lasting approximately 1 hour. During part of this scan, tDCS will be delivered. You will complete mood assessments with study staff, and complete some cognitive tests of thinking and memory on a computer. Time: Approximately 4 hours.
- Final Mood Assessments: 2 and 4 weeks after your final visit, you will receive a phone call from the study coordinator who will conduct a brief mood assessment at each timepoint.Time: Approximately 1 hour.
In total, if you are determined to be eligible for the research study and you consent to participating in the research study - you will receive 3 separate MRI scans and receive tDCS on 12 consecutive visits (excluding weekends). Your participation in this study will be complete after approximately two months.
For this study, you will be randomly assigned to a group of participants that will either receive active tDCS or in-active tDCS. Neither you nor the experimenter will know if you are receiving active stimulation because this information will be pre- programmed into the tDCS device based on a randomization code. This means if you agree to participate, you might receive in-active tDCS, which only mimics active stimulation. You will be told at the end of the study, that is, 4 weeks after your final visit and after your final mood assessment over the phone, if you received active or in-active tDCS. If you did not receive active tDCS during the research, you will be invited to receive the same number of active tDCS sessions after the study. You may decide or not decide to receive these additional active tDCS sessions after your research participation is complete.
Information about Transcranial Direct Current Stimulation (tDCS)
Below we include a summary of the medical and psychiatric uses of tDCS, potential adverse effects of tDCS, and major elements of the study protocol. Aspects of the study protocol will be implemented by UCLA research personnel, and not the patient’s psychiatrist or primary care physician.
tDCS uses in medicine and for treating depression:
- tDCS is a method of brain stimulation that uses electrodes placed on the scalp to deliver a constant, low current.
- tDCS has been used in many clinical research studies across the world over the last few decades, and has been safely investigated in depression, anxiety, chronic pain, and stroke.
- tDCS has been used as an experimental treatment for major depression, with evidence of efficacy described in some, but not all prior research studies1.
- There is never a guarantee of response and currently no way to predict who might respond.
Additional Resources to read more about tDCS for the treatment of depression:
- Medscape Expert Review of Neurotherapuetics By Drs. Colleen Loo and Donel Martin:
https://www.medscape.com/viewarticle/769494
Risks associated with tDCS:
- Potential risks and discomforts associated with tDCS include tingling, irritation, heating, or a light itching sensation under the electrodes. For many people, these sensations lessen or disappear soon after the start of stimulation, and are rarely reported as unpleasant.
- If you become too uncomfortable during the tDCS procedure, you can inform the staff who will immediately stop the tDCS session on request. If you feel uncomfortable tingling after the first 30 seconds of tDCS during the MRI, you can use the emergency squeeze ball provided to alert the study staff to stop the scan and tDCS procedure immediately. If you feel heating under the tDCS electrodes at any time, you are asked to inform the staff immediately.
- If your skin or scalp is currently irritated due to a pre-existing skin condition, treatment, or sensitivity, you must tell study staff at screening.
- tDCS uses a low level of electrical current and is not associated with risk of seizure. Nevertheless, people with a history of seizures and/or seizure disorder are excluded from this study.
Treatment procedure:
- Established safety precautions and parameters for tDCS will be strictly followed in this study.
- For this study, you will be randomly assigned to a group of participants that will either receive active tDCS or sham tDCS. Neither you nor the experimenter will know if you are receiving active stimulation because this information will be pre- programmed into the tDCS device based on a randomization code.
- For both active and sham tDCS, rubber electrodes will be placed on your scalp using gel or paste to improve electrical impedance and secured with elastic straps or a cap. These electrodes will have a 5 cm radius (HD tDCS).
- We will need you to refrain from making any drastic hair changes over the course of the study, in order to ensure that the electrode cap has a consistent fit throughout.
- During both active or sham tDCS, you might feel a mild tingling sensation under the electrodes that will lessen over about thirty (30) seconds. The mild intensity of the current delivered with tDCS can be compared to a flashlight powered by a 9-volt battery.
- The tDCS sessions will last for twenty (20) minutes.
- For the scans during which you will also receive tDCS, electrodes will be placed on your scalp and secured with elastic straps or a cap as above.
- These electrodes will remain on your scalp throughout the MRI experiment.
- The tDCS electrodes will be connected to a battery-driven constant direct current stimulator.
- At a certain time during the scan, the tDCS device will deliver a mild electrical current to the electrodes.
- You might feel a tingling sensation under the site of the electrodes that will diminish over time. This will feel the same whether you are receiving active or sham tDCS.
- You will receive a 30-minute structural imaging scan as part of your eligibility screening appointment, and an approximately 1-hour functional imaging scan during which you will receive tDCS for 30 minutes or less at the Baseline and Post-Trial visits.
References
- Palm, U., Hasan, A., Strube, W., Padberg, F. tDCS for the treatment of depression: A comprehensive review. Eur Arch Psychiatry Clin Neurosci 2016; 266(8): 681-694.
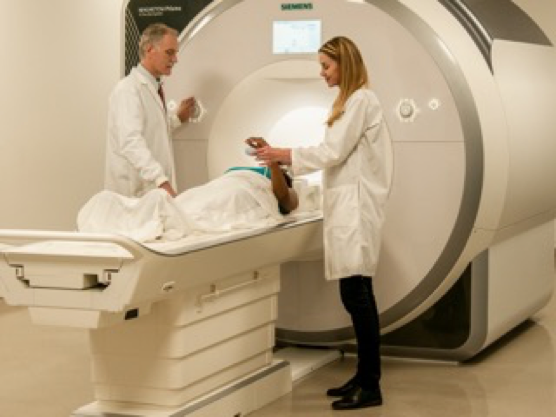
Magnetic Resonance Imaging (MRI) Brain Scan
Participation in this research study requires three separate MRI scans. Each MRI scan can last approximately 1 hour.
You cannot receive an MRI scan if:
- You have metal implants that contraindicate MRI scanning.
- You experience claustrophobia that would prevent you from staying inside a narrow, confined space for up to one and a half hours at a time.
- If female, you are pregnant or there is a possibility that you may be pregnant.
If you have metal implants, we require specific manufacturing information of these implants to determine MRI safety. Without this information, you will not be eligible.
Unlike a CT scan, a MRI scan does not use radiation. A MRI is essentially a big magnet that functions like a camera. For our study we are specifically looking at the brain. By taking pictures of your brain, we may better understand how tDCS affects brain function. The scans for our study can last approximately 1 hour. During that time, you will be lying flat and completely still while breathing comfortably during each scan. Your head and upper torso will be inside a large tube, and you will remain inside for the entire duration of the scan. In order to ensure you remain as comfortable as possible, the study team will check in with you in between each scan.
FAQ
- If I've been scheduled for my first consult visit, does that mean that I am eligible to receive tDCS as part of your research study?
- Why am I not eligible, and how can I receive tDCS if you have determined that I am not eligible?
- Who are the Principal Investigators of this study?
- Will I have access to the MRI data?
- Are there any weekend visits?
- What if I have additional questions about my rights as a research subject?
- I don’t live in Los Angeles, can I still be in the study?
- Will I receive compensation and transportation reimbursements?
- Will I need to take time off of work to participate in the study?
- Will I be able to reschedule any of the remaining study visits after I complete my baseline visit?
No. We are only able to enroll people who have a certain history of depression and who can undergo brain scans and other procedures needed for the research. A study physician will review information collected during the first consult visit to determine if you are eligible to participate in the research study.
We are only able to enroll people who have a certain history of depression and who can undergo brain scans and other procedures needed for the research. However, you may talk to your doctor about whether you might still be a candidate for tDCS for depression outside of this study.
This study is being conducted by Katherine Narr, PhD, and other co-investigators from the Departments of Psychiatry and Neurology at the University of California, Los Angeles (UCLA).
No, this information is only used for research purposes.
No, all of our in-person visits are scheduled on the weekdays.
Please call the UCLA Office Human Research Protection Program at (310) 825-5344.
Given the number of daily visits, you must live within driving distance of UCLA to enroll in the study.
You may receive up to $700 in cash and a $25 e-gift card for participating in this research study. You will receive $50 for each of the 13 research visits. You will receive payment after you have completed your first research visit (Consult), and payment for the remaining visits at the end of your final research visit (Post-Trial Assessment). You will receive a gift card for the two phone interviews. You will be reimbursed for parking at UCLA for each of the 13 research visits ($14 dollars each visit). Parking will be reimbursed at the time of each visit. Total payment over $600 will need to be reported to the IRS so your Social Security Number will be collected for this purpose only.
You will be able to return to work directly and resume your normal activities directly after research appointments.
No, your study visits are scheduled in advance and confirmed with you before your baseline visit. These visits are scheduled based off of strict study protocol and our limited availability.
Directions & Parking Information
If you have completed pre-screening by phone with a Study coordinator and have been scheduled for your initial visit to determine eligibility – below are directions for your first visit.
tDCS Study Location:
660 Charles E Young Dr South, Los Angeles, CA 90095
From the San Diego Freeway (405):
From the north, exit Wilshire East, or from the south, exit Wilshire at Westwood. Turn left on Westwood Blvd. and go straight past Le Conte Avenue (the street becomes Westwood Plaza as you enter the UCLA campus).
The closest parking lot to the Ahmanson-Lovelace Brain Mapping Center (BMC) is Lot 8. Parking permits are available for $14 and can be purchased on the top floor of the lot. You will be compensated for parking at the end of each visit.
Once you park at Lot 8, walk South on Westwood Plaza until you reach the cross street Charles E. Young Dr. S. Cross the street so that the Ronald Reagan Hospital is to your right.

Then, turn left (east) on Charles E. Young Dr. S, toward an incline.
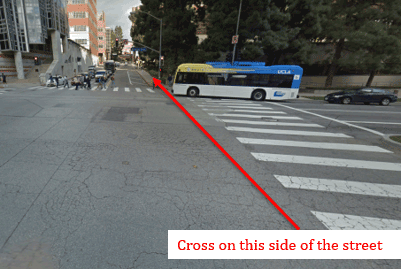
The BMC will be on your right. If you’ve reached Structure 9, you’ve gone too far. Once you’ve reached the BMC, please wait by the double door entrance and call the study coordinator to let her know you’ve arrived. A staff member will come out to greet you and your visit will begin.

Contact Us:
Please email DGCNeurostimStudy@mednet.ucla.edu or call 424-402-9051 to speak to the Study Coordinator.